Published on 2024-05-28

Table of Contents
What Is Contract Medical Device Manufacturing?
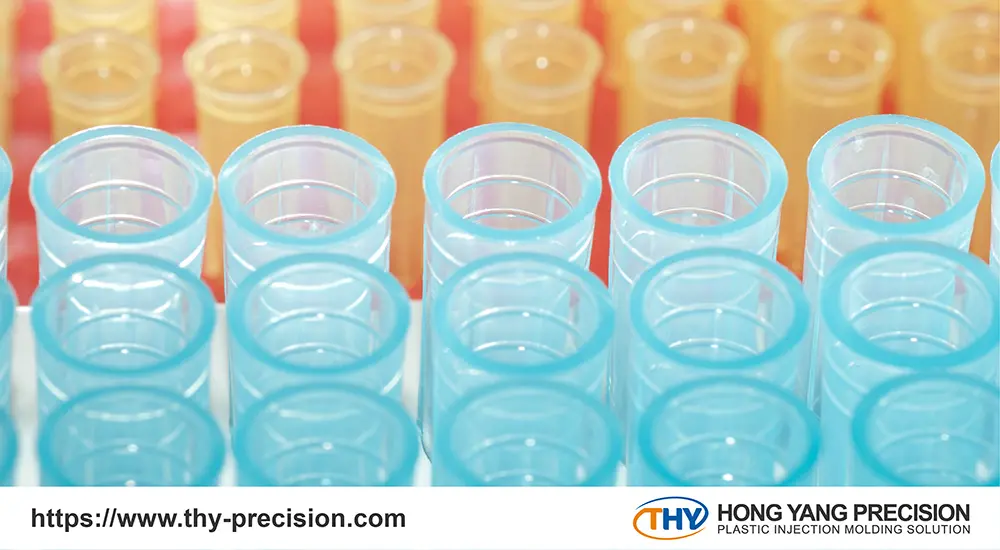
Medical device contract manufacturing has become an integral part of the healthcare industry’s supply chain. These specialized firms leverage their expertise and production capabilities to manufacture medical equipment on behalf of original equipment manufacturers (OEMs). Rather than handling all aspects of device production internally, OEMs can outsource manufacturing to these contract partners.
The role of contract medical device manufacturers is to translate a customer’s product designs and specifications into high-quality, compliant devices. They possess the specialized equipment, validated processes, and regulatory know-how required to produce Class I, II, and III medical products. Beyond just manufacturing, they often provide additional services such as packaging, labeling, and sterilization to deliver a turnkey supply solution.
What Does a Contract Medical Device Manufacturer Do?
For OEMs, collaborating with a contract medical device manufacturer unlocks several strategic advantages. It allows them to focus on core competencies in R&D and product innovation, while streamlining their supply chain.
Contract manufacturers also help OEMs meet strict delivery timelines, control production costs, and ensure their devices adhere to all relevant industry standards. This symbiotic partnership enables OEMs to bring safer, more reliable medical technologies to market more efficiently.
Ensure Quality Compliance
Ensuring quality compliance is paramount for medical device contract manufacturers. Lives could depend on their products, so preventing quality issues is a critical responsibility. These specialized firms thoroughly inspect and assess every item throughout the production process, using sophisticated measurement equipment to quickly identify and address any defects.
Beyond individual product quality control, contract medical device manufacturers are also tasked with continuously improving their production processes. They must adhere to ISO 13485 guidelines, which validate the efficacy of their quality management systems and affirm the safety and efficacy of the manufactured parts.
By upholding these stringent quality standards, medical device contract manufacturers ensure the devices they produce meet all regulatory requirements and can be trusted to perform reliably in clinical settings.
Streamlined Operations and Cost Savings
Medical device contract manufacturers provide OEMs with a turnkey production solution. They can handle the full spectrum of manufacturing responsibilities – from facilities and equipment to staffing and logistics. Whether for prototypes or large-scale rollouts, contract manufacturers shoulder these upfront burdens.
Beyond just production, these firms manage the ancillary services required to commercialize medical devices, like packaging, welding, decorating, and sterilization. By handling this end-to-end process, contract medical device manufacturers free OEMs to focus on innovation and enhancing patient care.
Outsourcing production allows OEMs to avoid capital-intensive investments and operational complexities. This enables greater agility, responsiveness, and cost-efficiency in bringing safe, effective medical technologies to market.
How to Choose a Contract Medical Device Manufacturer?

Choosing the right contract medical device manufacturer can be a complex decision for original equipment manufacturers (OEMs). These specialized companies play a vital role in the medical device manufacturing process, handling a range of services from casting and molding to R&D design and custom packaging. When seeking the right medical contract manufacturer to partner with, medical instrument companies must consider the key factors that will ensure a successful long-term collaboration.
High-Quality Manufacturing with ISO 13485 Certification
A high-quality system and ISO 13485 certification are invaluable for medical contract manufacturers working with original equipment manufacturers (OEMs). This internationally recognized standard holds contract manufacturers to rigorous requirements, ensuring they consistently meet regulatory standards and satisfy their customers’ needs. Achieving ISO 13485 compliance demonstrates that the contract manufacturer has invested the time, effort, and resources to establish quality systems that are equivalent or exceed industry benchmarks.
Quality is often the most critical factor in decisions involving medical devices. Choosing a contract manufacturer with the necessary ISO 13485 accreditation and a strong focus on quality can save OEMs time and money in the long run. These credentials provide assurance that the contract manufacturer has the expertise and capabilities to deliver high-quality medical products that meet all regulatory requirements.
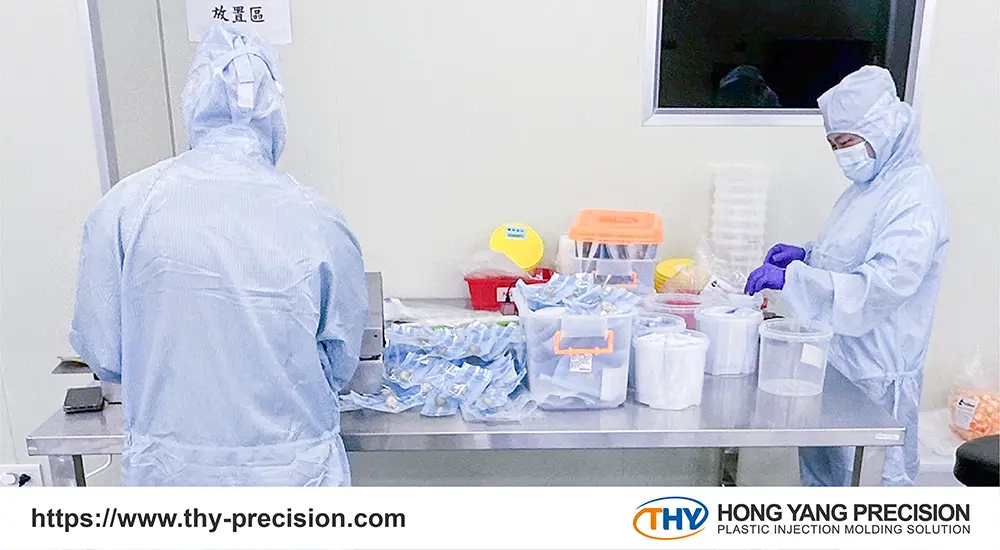
Cleanroom Capabilities
Selecting a contract medical device manufacturer with cleanroom capabilities offers significant advantages for original equipment manufacturers (OEMs). Devices fabricated in a cleanroom environment have a lower risk of contamination, as the entire production process is treated as if the product requires sterile handling. This results in better quality devices, as the stringent cleanroom protocols and specialized equipment ensure a high level of cleanliness and care throughout manufacturing.
Additionally, contract manufacturers with cleanroom facilities typically employ highly skilled technicians who are meticulously trained to maintain the controlled environment and handle the devices with the utmost diligence. This specialized expertise and attention to detail during the manufacturing process provides OEMs with added assurance of product quality and reliability.
Right Machines and Equipment

When choosing a contract medical device manufacturer, it’s crucial to ensure they have the right machines and equipment to produce your product effectively. The capabilities of their machinery and facility directly impact their ability to meet your specific manufacturing requirements.
Does the contract manufacturer have the necessary molding, machining, or processing equipment? Do they have the production capacity and clean room facilities needed to maintain quality standards? These are essential considerations, as the right machinery and infrastructure are fundamental to their performance.
Selecting a partner with the appropriate equipment means they can execute your product design with precision and efficiency, minimizing quality issues or delays. Thoroughly vetting a potential contractor’s technical capabilities is key to a successful outsourcing partnership.
Manufacturing Efficiency

The adoption of automated manufacturing is transforming medical device production. Automated processes optimize application performance, improve efficiency and quality, and reduce human error. This approach also enables part consolidation, streamlining assembly, and lowers long-term installation costs by increasing efficiency.
When selecting a contract manufacturer, it is crucial to understand their use of automated technologies. A contractor’s automated manufacturing capabilities can directly impact the quality, turnaround time, and cost-effectiveness of your outsourced operations. Thoroughly vetting their expertise in this area is essential for securing an efficient, reliable supplier.
Conclusion

Contract medical device manufacturers have become indispensable partners for healthcare OEMs. These specialized firms possess the expertise, equipment, and regulatory know-how to transform product designs into high-quality, compliant devices. By outsourcing production, OEMs can streamline operations, reduce costs, and focus on innovation.
When selecting a contract manufacturer, evaluate factors like quality certifications, cleanroom capabilities, machinery, and automation. Choosing the right partner with these essential capabilities will ensure your devices meet regulatory requirements and deliver the performance your customers demand.
To explore how THY Precision’s contract medical device manufacturing services can benefit your medical device business, contact us today. Our experts are ready to deliver a tailored, turnkey solution that drives efficiency and quality throughout your production.
Learn more:




